Confocal Imaging and Analysis Using the Agilent BioTek Cytation C10
Since 2012, cells and tissues from many sources have been cultured in three dimensions (3D), enabling more complex biological models. The use of these 3D models has been increasing in medical research, precision medicine, disease modeling, and drug discovery efforts.
These 3D models are being used to simulate the native microenvironments found in organisms and are believed to provide a more accurate assay model in some instances. 2D models, consisting of a monolayer of cells, have been in use for decades and have provided extensive meaningful data.
However, 2D limitations became more apparent with the recent development of complex organoids, tissues, or tumoroids. These tissues, comprised of one or more cell types and often based on stem cells or patient-derived samples, have now become the focus of many studies.
The Cytation C10
The ability to perform a variety of assays on a single, compact instrument is advantageous, especially given the limited bench space in many laboratories. The Agilent BioTek Cytation C10 confocal imaging reader can perform microplate reading, widefield imaging, and confocal imaging, offering the means to gather data for today’s complex studies.
The Agilent application note “Confocal Imaging and Analysis of Spheroids for Determination of Dose Response During Drug Treatment” by Peter J. Brescia describes the use of automated imaging to determine cell number in spheroids to evaluate drug dose responses.
Study Materials and Methods
The study discussed in the application note used CT116-H2B-GFP cells; the H2B-GFP construct provided a constitutively expressing nuclear marker for quantification by cell count for comparison to a nuclear stain during live cell analysis.
The cells were seeded at a relatively low density of 500 cells/well in a 96-well, round bottom ULA imaging plate (Cat. No. 15-100-173) and grown for three to four days to produce spheroids of roughly 100 µm in size.
Staurosporin, a potent, nonselective protein kinase inhibitor, was added to induce apoptosis. Propidium iodide (PI) was also added to monitor the kinetics to determine optimum timing (12 hours post-addition produced adequate apoptotic activity for analysis).
Study Imaging Procedure and Analysis
To minimize the data set, spheroids were sized to be captured in a single field of view (20X objective) and a beacon was used to correct for positioning variability in the wells. A 60 µm pinhole spinning disk provided a compromise between acquisition time and signal intensity for confocal imaging. Widefield images were captured in both GFP and TRITC channels to compare to confocal images.
The instrument was set for “Scan,” followed by “Autofocus,” which identified the approximate center of the spheroid along the z-axis. Each spheroid was captured as a z-stack using 11 steps at 10 µm, regardless of the z position in the well. Images were processed in several steps, including a z-projection for comparing single z-plane images at the approximate center of the spheroid and the entire z-stack for both imaging modes.
Automated cell counting was performed using confocal and widefield imaging mode and both single plane and z-stack projected images were analyzed.
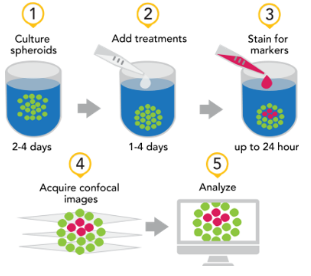
Figure 1. Spheroid assay workflow incorporating automated image acquisition and analysis (Agilent BioTek Cytation C10 confocal imaging reader and Agilent BioTek Gen5 microplate reader and imager software).

Figure 2. Spheroid captured as a z-stack using 11 steps at 10 µm intervals. Analysis performed on either a z-projection of all images or a single image representing a plane through the center of the spheroid.
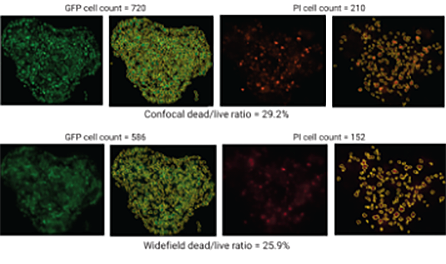
Figure 3. Examples of automated image acquisition and cell count analysis of spheroids by confocal and widefield fluorescent microscopy using z-projected images. Cell masking (yellow outlines) where GFP cell count indicates live cells and PI indicates dead cells.
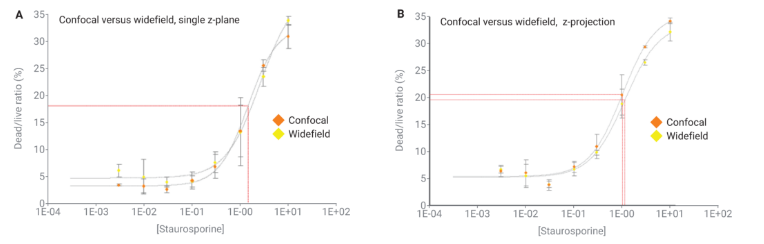
Figure 4. EC 50 determinations for each imaging mode, single versus z-projected images.
Since spheroids are increasingly used to represent the complex microenvironments of biological systems, the ability to automate image capture using multiple imaging modes and perform automated image analysis can increase throughput and enable comparative analysis.
The study results confirmed that confocal optics enable more accurate segmentation of labeled cells within spheroid samples than widefield optics. Cell count determination using a single z-plane image provided comparable results to methods relying on z-stack image acquisition and processing.
The study is just one example of the utility and flexibility of the Agilent BioTek Cytation C10 confocal imaging reader and Agilent BioTek Gen5 microplate reader and imager software to capture and analyze multiple parallel data sets.

Content provided by: