Vaccine Manufacturing
Cleanroom Apparel to Protect Processes, Products, and Operators
Vaccine manufacturing has been of paramount importance during the COVID-19 pandemic and remains a fast-growing market sector. The manufacturing processes for vaccines are complex and consist of many steps. Most vaccine manufacturing processes depend on quality control at every step. Failures can be costly, dangerous, and may compromise compliance.
Cleanroom garments protect both processes and products from contamination while protecting operators from the hazardous substances involved in pharmaceutical manufacturing. Find out how DuPont Tyvek and Tyvek IsoClean coveralls and accessories can be beneficial to successful vaccine manufacturing.
Operators and Cleanroom Contamination
Operators are the main source of contamination in cleanrooms. While contamination cannot be wholly eliminated, levels can be significantly reduced through training and impeccable hygiene.
The proper use of cleanroom garments can prevent particle contamination by acting as a barrier between operators and the production environment. The 2020 draft of the Good Manufacturing Practice (GMP) guidelines Annex 1* states that “cleanroom garments should retain particulates shed by the body.” Sufficient cleanroom clothing is therefore required during most steps of the vaccine manufacturing process to prevent contamination, maintain patient safety, and protect employees.
Advantages of Tyvek Fabric
For over 20 years, Tyvek and Tyvek IsoClean garments have been used extensively in vaccine manufacturing because of their fabric, design, and dependable performance. Tyvek is made from high-density polyethylene filaments that are thermally bonded into a tight, homogeneous, and soft breathable fabric with low-linting and strong barrier properties. This unique combination of barrier protection and breathability makes Tyvek suitable for cleanroom environments and meets GMP standards. Additionally, Tyvek fabric offers operator protection against chemicals and biological substances.
Cleanroom and Production Protection
- Suitable for ISO Class 4-9, GMP A/B, C/D, and other cleanroom types
- Operator-generated contamination barriers (bacterial and particle filtration efficiency)
- Low particle release
- Available in clean-processed and sterile options
Operator Protection
- Repels aqueous liquids and liquid aerosols
- Provides biological barrier protection
- Two-way particle barrier
- Tear- and abrasion-resistant
Operator Comfort
- Lightweight and soft
- Breathable
- Apparel designed based on workplace demands
- Specific donning and doffing procedures
Compliance with QRM and GMP Annex 1
GMP Annex 1 (2020 draft) anticipates that all pharmaceutical manufacturing activities will be governed by quality risk management (QRM) principles as documented in the contamination control strategy (CCS).
This proactive approach means it is not enough to simply react to and correct detected contamination. Manufacturers will be expected to identify potential quality risks and implement technical and procedural means to control risk and enact continuous improvements.
Cleanroom garment systems are a critical part of sterile and aseptic manufacturing. They must be managed under QRM principles to ensure GMP compliance and, ultimately, patient safety. Vaccine manufacturing involves a number of manual interventions that can result in a risk to operators. Manufacturers are required by the Occupational Safety and Health Administration (OSHA) to equip operators with appropriate PPE based on the assessment of risk to their health and safety.
Consistent Performance
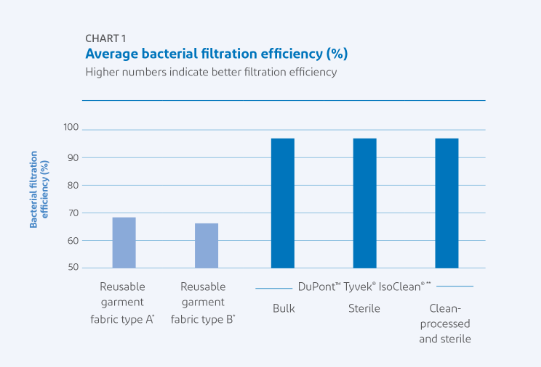
Regulators expect vaccine manufacturers to keep vaccines free from contaminants at all times, and operators rely on the barrier performance of their cleanroom garments. Tyvek IsoClean sterile cleanroom garments make contamination control easier. Since IsoClean garments are used only once, they offer consistent performance in Helmke drum tests, particle filtration efficiency, and bacterial filtration efficiency. Reusable cleanroom garments that are washed, dried, and sterilized multiple times vary in performance, which can deteriorate with time and repeated use.
Also, the effects of gamma radiation on the polymer occurs just once for single-use garments, so their properties are more consistent. The average bacterial filtration efficiency of reusable garments is in the 64 to 69 percent range, while the average bacterial filtration efficiency of Tyvek IsoClean single-use garments is typically 98 to 99 percent.
Peace of Mind
As the manufacturer of both the Tyvek material and sterile IsoClean garments, DuPont controls the supply chain and can provide test data and lot-based certificates of sterility, irradiation, and compliance at multiple points during apparel manufacturing. Qualification and subsequent quality audits can be easier than with other cleanroom garments that involve several different PET filament manufacturers, fabric weavers, garment manufacturers, and laundries. Managing levels for single-use Tyvek IsoClean garments can also be easier than managing reusable garments that require washing, sterilizing, disinfecting, garment repair and replacement, multiple invoices, and other management steps.
A Flexible Solution
Vaccine manufacturing companies continue to grow and expand, and manufacturing contracts are rarely synchronized with laundry contracts. Using disposable Tyvek coveralls can offer more flexibility, speed production, and eliminate laundry processes.
Single-use garments offer maximum flexibility and protection for start-ups, small-batch production, single-use reactor production, and manufacturing that requires frequent adaptation and changes. Additionally, Tyvek and IsoClean garments that have not been exposed to hazardous substances can be recycled using the DuPont Tyvek Protective Apparel Recycling Program.
Protect your processes, products, and operators through each step of vaccine manufacturing with single-use, GMP-compliant protective products that offer consistent and reliable performance.
*The EU GMP Annex 1 revision for the manufacturing of sterile medicinal products was finalized and published on August 25, 2022, and is set to go into effect on August 25, 2023.




