Metathesis Method in Organic Synthesis
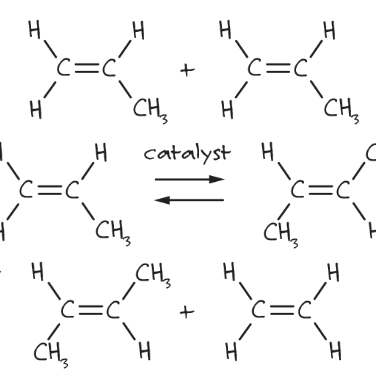
The term “metathesis” is used in linguistics and occurs when two sounds or syllables change place in a word. Why is this term now often heard in the scientific world? What is metathesis in chemistry, and why has it become so important? Let’s start with the example reported below to explain it.
Scheme 1. Example of metathesis
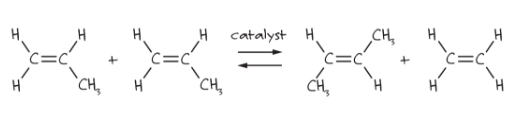
Scheme 1 is one of the basic types of metathesis, where two double bonds are broken and two new ones are formed in the presence of a catalyst. Metathesis was first observed in the 1950s and was used in industry for the polymerization of olefins, but the catalyst’s function was uncertain. In the first hypothesis, a π-cyclobutane-metal intermediate was formed in the mechanism. Finally, in 1971, Yves Chauvin and Jean-Louis Hérisson proposed the role of a metal carbene to initiate the reaction.1 After many years, we can now describe the mechanism as reported in Scheme 2.
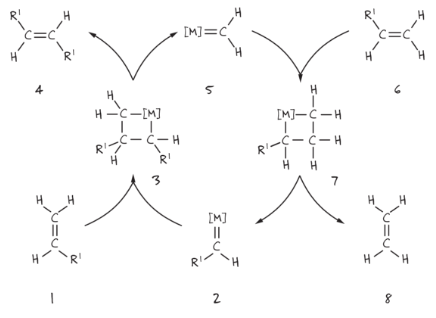
Scheme 2. Mechanism proposed by Chauvin and Hérisson
In the first step, alkene 1 reacts with the metal carbene 2 to give cyclic intermediate 3; single bonds are broken to form the product alkene 4 and the new metal carbene 5. The latter reacts with the second alkene 6 to give the new metallocyclobutane 7 that will reform the starting metal carbene 2 and produce ethylene 8.
The reaction shown in Scheme 2 is known as cross metathesis (CM) when two different olefins are involved. Other types of metathesis are:
- Ring Opening Metathesis Polymerization (ROMP), where cyclic olefins are opened to give a corresponding polymer
- Ring Closing Metathesis (RCM), the opposite of ROMP, where two double bonds in the same molecule react to give a cyclic olefin and ethylene
- Acyclic Diene Metathesis Polymerization (ADMET), where acyclic dienes polymerize to give a polyene
Over the years, many researchers were actively involved in the study of metathesis reactions, and in 2005, Yves Chauvin, Richard R. Schrock, and Robert H. Grubbs were recognized for their important contributions with the Nobel Prize in Chemistry “for the development of the metathesis method in organic synthesis.”2
Firstly, Schrock tried different metal catalysts and, in 1990, proposed a molybdenum catalyst 9 for olefin metathesis.3 Schrock worked later with A. H. Hoveyda to synthesize a chiral catalyst for catalytic asymmetric metathesis.4 Then, in 1992, Robert Grubbs discovered the first ruthenium-based catalyst 10 with a higher selectivity but a lower reactivity than molybdenum catalyst 9.5 Subsequently, Grubbs developed the airstable catalyst 11, which became known as the first generation Grubbs catalyst, a real milestone in the development of this type of chemistry.6
The first stage of the proposed reaction mechanism (for catalyst 11) involves the dissociation of one of the phosphine ligands to give a 14-electron intermediate.7 In general, the faster the rate of phosphine dissociation, the higher the catalyst activity. This is an important consideration when designing new ruthenium-based metathesis catalysts. Two factors that promote phosphine dissociation are:
- Using electron-donating (nonhalide) ligands that stabilize the 14-electron intermediate
- Use of bulky ligands that crowd and destabilize the complex
These characteristics are typical of the N-heterocyclic ligands that were used in the second-generation Grubbs catalyst to replace one tricyclohexylphosphine (compound 12).8 Although compound 12 demonstrated a significantly higher metathesis activity, it was observed that phosphine dissociation was slower than for compound 11. The improvement in the activity of 12 was attributed to a higher affinity for the olefin substrate compared to catalyst 11 after dissociation had occurred.
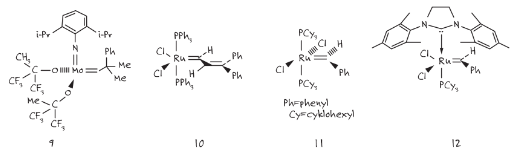
Image 1. Schrock (9) and Grubbs (10,11,12) catalysts.
Grubbs’ research continued with the development of further ruthenium-based catalysts that established metathesis chemistry’s role in several commercial applications.
The second Generation Hoveyda-Grubbs Catalyst, for example, has similar activity to the second Generation Grubbs Catalyst, but it initiates the reaction at lower temperatures and is easier to store.
Metathesis has played and continues to play an important role in academic research and is used in pharmaceuticals and biotechnology applications and in developing polymeric materials. Because the literature describes so many metathesis applications, it is not possible to list all of them here (some examples are the coupling of olefins with α,β-unsaturated carbonyls, or the synthesis of α,β-unsaturated amides).9,10
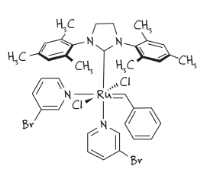
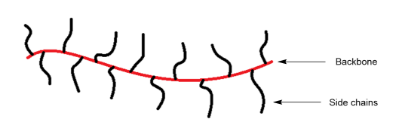
Image 2. Grubbs third generation catalyst (on the left) and a scheme of a bottlebrush (on the right)
An interesting example is the synthesis of bottlebrush copolymers using the ROMP method and the Grubbs third generation catalyst (Image 2).
A bottlebrush polymer consists of a main polymeric chain (backbone) containing polymeric side branches.11 Two, or more, different side chains can be attached to a backbone providing access to copolymers with unique architectures that possess novel and potentially useful properties. The main chains are usually norbornene polymers due to the high polymerization rate and commercial availability of the monomers. Poly(ethylene oxide) side chains have been used for drug delivery studies, tumor targeting, and in battery research.
- Herisson J. L. & Chauvin Y. (1971). Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d’oléfines acycliques. Die Makromolekulare Chemie, 141, 161-176. https://doi.org/10.1002/macp.1971.021410112
- The Royal Swedish Academy of Sciences. (2005, October 5). The Nobel Prize in Chemistry 2005. Retrieved from https://www.nobelprize.org/prizes/chemistry/2005/press-release/
- Schrock, R.R., Murdzek, J.S., Bazan, G.C., Robbins, J., Dimare, M., O’Regan, M.J. (1990). Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. Am. Chem. Soc., 112(10) 3875-3886. https://doi.org/10.1021/ja00166a023
- Hoveyda, A.H. & Schrock, R.R. (2001). Catalytic Asymmetric Olefin Metathesis. Chem. Eur. J., 7(5) 946-950. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/1521-3765(20010302)7:5%3C945::AID-CHEM945%3E3.0.CO;2-3
- Nguyen, S.T., Johnson, L.K., Grubbs, R.H., Ziller, W. J. (1992). Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media. Am. Chem. Soc., 114(10), 3974-3975. https://doi.org/10.1021/ja00036a053
- Schwab, P., France, M.B., Ziller, J.W., Grubbs, R.H. (1995). A Series of Well-Defined Metathesis Catalysts–Synthesis of [RuCl2(=CHR′)(PR3)2] and Its Reactions. Angewandte Chemie International Edition in English, 34(18), 2039-2041. https://doi.org/10.1002/anie.199520391
- Sanford, M.S., Love, J.A., Grubbs, R.H. (2001). Mechanism and Activity of Ruthenium Olefin Metathesis Catalysts. J. Am. Chem. Soc., 123, 6543- 6554. https://doi.org/10.1021/ja010624k
- Scholl, M, Trnka, T.M., Morgan, J.P., Grubbs, R.H. (1999). Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Letters, 40(12), 2247-2250. https://doi.org/10.1016/S0040-4039(99)00217-8
- Chatterjee, A.K., Morgan, J.P., Scholl, M, Grubbs, R.H. (2000). A General Model for Selectivity in Olefin Cross Metathesis. J. Am. Chem. Soc., 122, 3783. https://doi.org/10.1021/ja0214882
- Choi, T.L., Chatterjee, A.K., Grubbs, R.H. (2001). Synthesis of α,β-Unsaturated Amides by Olefin Cross-Metathesis. Angew. Chem. Int. Ed., 40(7), 1277. https://onlinelibrary.wiley.com/doi/10.1002/1521-3773(20010401)40:7%3C1277::AID-ANIE1277%3E3.0.CO;2-E
- Choinopoulos, I. (2019). Grubbs’ and Schrock’s Catalysts, Ring Opening Metathesis Polymerization and Molecular Brushes-Synthesis, Characterization, Properties and Applications. Polymers, 11(2), 298, 2-31. https://doi.org/10.3390/polym11020298