Air-Sensitive Chemistry: Practical and Safety Considerations
Air-sensitive chemicals are commonly used in academic and industrial chemistry laboratories. Examples of air-sensitive reagents include organometallic compounds like organo-lithium, -magnesium, -zinc, and -aluminum; hydrides; borane complexes; and alkaline metals and several transition metals in their (0) oxidation state. Some of these chemicals are also pyrophoric and spontaneously ignite under standard conditions when they come into contact with air.
Many incredibly useful synthetic reactions are air sensitive, including Grignard reactions, reductions using hydrides, metalations and transmetalations, carbolithiation, and many more. These reactions are widely used to synthesize fine chemicals, drugs, polymers, and many other products.
The exposure of these reactions to air, oxygen, or moisture can:
- Favor side-reactions that produce undesired products
- Decompose reagents so that no reaction occurs
- Cause fires, explosions, or other hazardous conditions
Handling Air-Sensitive Reactions
Air sensitivity can be separated into two categories: catalytically and stoichiometrically driven.
A typical example of catalytic air sensitivity, reported in Figure 1a, is the ignition of residual organic solvents or H2 gas adsorbed on the surface of a reduced Pd (0) catalyst, because of its exothermic oxidation in the presence of atmospheric oxygen. Stoichiometric air sensitivity is very common for many organometallic species, such as the formation of butane gas by reaction of buthyl lithium with water, illustrated in Figure 1b, with strong release of heat that can easily cause a fire.
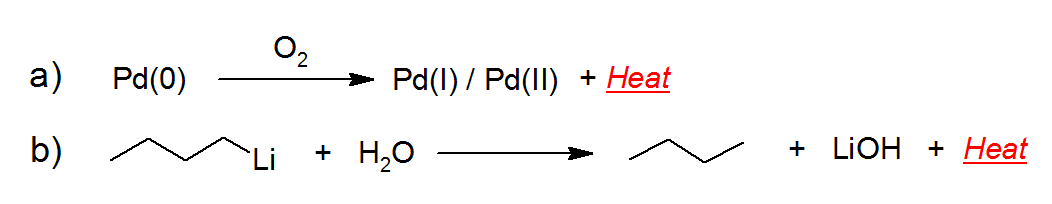
Handling catalytic air sensitivity requires specialized laboratory apparatus: vacuum lines, Schlenk lines, inert-atmosphere glove boxes, and special reactors. Most of the synthetically useful compounds, however, belong to the stoichiometric category and are frequently used reagents by the majority of chemists.
Extra-dry solvents are another class of air- and moisture-sensitive products, but they do not present intrinsic hazards. In their case, inappropriate handling and storage can cause the product to degrade over time. This is hard to detect but can interfere with experiments, affecting results and often requiring time-consuming troubleshooting.
Laboratory Safety Considerations
While the contact of an extra-dry solvent with moisture may not itself be a hazard, using a water-contaminated solvent in air-sensitive reactions can lead to very dangerous situations. Extra-dry solvents would for this reason be stored with the same care as pyrophoric compounds or solutions and other hazardous substances.
These considerations are very important because laboratory safety is a high priority for industrial and academic researchers alike. During the past fifteen years, there have been multiple high-profile accidents, several of which have been summarily attributed to the inexperience of students working in academic labs. Accidents can rarely be attributed to a single cause, and further investigation of these incidents reveals several contributing factors.
There are no comprehensive datasets about the types and frequency of accidents and near-misses, which makes meaningful laboratory safety research difficult, according to Dana Menard and John F. Trant’s review published in Nature Chemistry in 20201. Available data, however, show a high incidence of accidents involving reactive gases and pyrophoric materials. Butyllithium, for example, is known to cause problems in academic organic chemistry labs where correct management of air sensitivity can often be an issue.
The presence of more experienced research personnel in industrial and government laboratories may make these labs somewhat safer. However, a study by Schroder and associates2 found that standardized risk assessments were not routinely used. Industrial and government labs reported higher compliance rates than the average in academic environments, but the average remains worryingly low at 43% and 36% respectively.
Safely Handling Air- Sensitive Chemicals
The use of air-sensitive reagents in chemical research is not new. A significant amount of literature is available to provide foundations for safe handling and operation3. An appropriate risk assessment and procedure must be based on the following two practices:
- The use of clean and dry glassware and equipment
- The use of specialized packaging, syringes, and inert dry gases
While the first practice is straightforward, it must be taken seriously. Reagents can react violently in the presence of even tiny amounts of water, and minor air moisture condensation caused by temperature differences between labware and the environment can be enough to cause a fire. The second practice requires a different type of attention. Many air-sensitive reagents and ultra-dry solvents and solutions are specially packaged to help simplify their handling.
AcroSeal Packaging
The Acros Organics and Alfa Aesar brands offer AcroSeal packaging, the industry-leading solution. Its multi-layer septum and innovative anti-tampering cap (with ample surface area) provide convenient and safe storage and dispensing while limiting exposure to the atmosphere. Syringes pass easily through the septum and create only a small hole that self-heals quickly under normal circumstances.
To dispense chemicals from AcroSeal packaging, use a syringe with an 18- to 21-gauge needle and a dry inert gas like nitrogen or argon. First, pressurize the bottle by injecting the gas and then withdraw the desired amount of liquid. Alternatively, use a double or double-tipped needle — one needle to withdraw the liquid and the other to add the inert gas from a gas line or balloon.
Best Practices
Some protocols recommend using glass syringes for pyrophoric reagents, but recent studies4 suggest that less experienced users may find it easier to substitute a single-use polypropylene Luer lock syringe.
Drying solvents or preparing solutions of air-sensitive reagents can be tedious and time-consuming, and it often presents significant hazards. Ready-made products in specialized safe packaging provide a convenient, cost-effective, and safety-oriented solution.
The AcroSeal technology has undergone several innovations during its 25 years on the market. More than 2,000 Acros Organics and Alfa Aesar products are currently available in AcroSeal packaging.
References:
- A.D. Ménard and J.F. Trant, A review and critique of academic lab safety, Nature Chemistry 2020, 12, 17–25
- Schröder, I., Huang, D. Y. Q., Ellis, O., Gibson, J. H. & Wayne, N. L. Laboratory safety attitudes and practices: A comparison of academic, government, and industry researchers. J. Chem. Health Saf. 2016, 23, 12–23
- D.F. Shriver, M.A. Dredzdon, The manipulation of air sensitive compounds, John Wiley & Sons, 1986
- E.S. Von Nehring, V. Dragojlovic, Handling of Air-Sensitive and Moisture-Sensitive Reagents in an undergraduate Chemistry Laboratory: The Importance of the Syringe, J. Chem Ed. 2021, 98, 246-249





