Neuroprotection and Neurodegeneration
Microglia in Acute and Chronic Inflammation
By Chandra Mohan, PhD, MilliporeSigma, Temecula, CA
The term neurodegeneration characterizes a chronic loss of neuronal cell structure and function leading to progressive mental impairments. Generally, the incidence of neurodegeneration increases with age, but infectious agents can also induce neuronal death via apoptosis. Research has linked neurodegeneration to neuroinflammation, which can be caused by microvascular damage, atherosclerosis, age-associated pro-inflammatory signals, and viral or bacterial infections that compromise the blood-brain barrier.
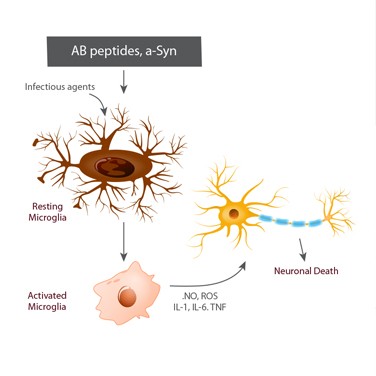
Most neurological disorders or damage causes inflammation, which can cause microglia cells to increase in number and change phenotypes. Microglia are a type of cell that is found throughout the brain and spinal cord and are the “first responders” in the active immune defense of the central nervous system. They protect against potential fatal damage by scavenging plaques, damaged or superfluous neurons and synapses, and infectious agents.
Microglia may comprise up to 15% of all brain cells and play a crucial role during brain development: They induce apoptosis of specific neuronal subpopulations and contribute to numerical control of neuronal cells. These cells also support synaptogenesis through the local synthesis of neurotrophic factors, and regulate synaptic transmission and remodeling. However, microglia may be detrimental to neurons when they establish a chronic inflammatory function and promote neuropathologies.
Acute neuronal damage that results from stroke, hypoxia, and neurotrauma can also compromise neuronal survival and indirectly trigger neuroinflammation. In these latter cases, microglia are activated in response to the insult and adopt a phagocytic phenotype. They release inflammatory mediators such as cytokines and chemokines, which may be considered a protective response to limit further injury and initiate repair processes.
Neurodegenerative diseases — including Alzheimer’s and Parkinson’s diseases and multiple sclerosis — cause chronic neuroinflammation that persists long after the initial injury or insult and results in long-standing activation of microglia and sustained release of inflammatory mediators. These mediator molecules increase oxidative stress and perpetuate the inflammatory cycle, activating additional microglia, promoting their proliferation, and causing the release of more inflammatory factors. With this chronic neuroinflammation, microglia activation has detrimental effects and forms the basis of the microglial dysfunction hypothesis.
During Alzheimer’s disease progression, the activation of microglia can either reduce the levels of Aβ peptides via phagocytosis, clearance, and degradation, or release pro-inflammatory cytokines that can contribute to neuronal damage and loss. Under physiological conditions, active microglia generally maintain homoeostasis and play a role in neuroprotection and repair by releasing growth factors, such as BDNF and TGF-b. However, under pathological conditions like infection, injury, and ischemia, they are activated, proliferate, and assume a macrophage-like morphology.
Activated microglia can produce both neurotoxic and neuroprotective effects. For example, microglia activated by lipopolysaccharides, IFN-g or TNF-a, are known as “M1” or “classically activated” and play a vital role in defense against pathogens and tumor cells by producing pro-inflammatory cytokines, such as IL-1β, TNF-α, and free radicals. The alternative “M2” type of microglia have an anti-inflammatory phenotype that promotes tissue remodeling and repair and angiogenesis via the release of antiinflammatory cytokines IL-4 and IL-10.
In murine models of Alzheimer’s disease, increased numbers of both M1 and M2 microglia have been reported (compared to age-matched control animals) and microglia can switch from M2 to M1 type with the progression of disease. NALP3 inflammasome, a mediator of IL-1β production, plays an important role in driving the innate immune response towards Aβ peptides and blocking NALP3 activity, which can induce microglial phagocytosis and a shift towards the M2 phenotype.
An early expression of pro-inflammatory cytokines by non-neuronal (including endothelial) cells in brains affected by Alzheimer’s may also affect disease development. Compared to age-matched controls, microvessels in these brains can release high amounts of pro-inflammatory cytokines. In addition, sustained inflammation from the periphery may contribute to the brain uptake of proinflammatory cytokines, mediated by receptors for advanced glycation end products (RAGE) and a compromised blood-brain barrier.
Increasing evidence supports the role of cytokines and other mediators in neuroinflammation and neurodegradation. Newer therapeutic approaches for treatment of stroke and debilitating neurodegenerative diseases may depend upon a better understanding of microglial physiology and the ability of resident microglia to initiate proinflammatory immune responses.