Pretzel-Shaped Molecules Could be the Future of Antibiotics
By Mike Howie
Chemists from the Van ‘t Hoff Institute for Molecular Sciences at the University of Amsterdam took a major step toward synthesizing lasso peptides earlier this year by creating a new class of molecules called quasi[1]catenanes. The new molecules are shaped like nanoscopic pretzels, with two molecular rings oppositely coupled at a central carbon atom. PhD student Luuk Stemers led Professor Jan van Maaseveen’s Synthetic Organic Chemistry research group in the study, which took five years to complete and was published in Nature Communications in May 2017.
Lasso peptides, first discovered more than 25 years ago, are small ribosomally assembled and post-translationally modifi ed strings of amino acids (RiPPs) that act as antibiotics in bacteria, protecting them against other microorganisms. Although they have the potential to be used as antibiotics in humans, their structural complexity has prevented chemists from developing a strategy to reliably synthesize and replicate their molecular architecture.
Synthesizing the Structure
Lasso peptides consist of a “rope” or tail (C-terminal) with one end enclosed in a “loop” (a macrolactam ring at the N-terminal) so that it resembles a lariat or slipknot. The loop is kept in place by two residues attached above and below the ring. The structure itself is extremely resistant to thermal, chemical, and proteolytic degradation, making it a promising option for therapeutic peptide applications.
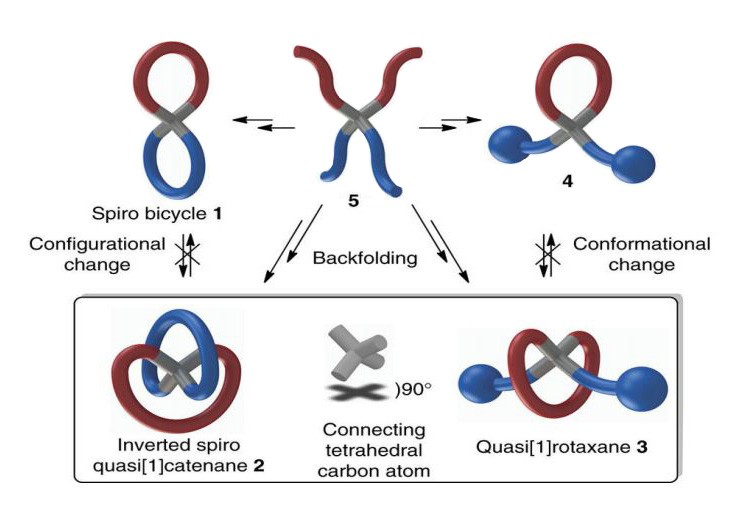
In an attempt to recreate the unique “lasso” structure, the research team wanted to force the loop to close around the tail in precisely the right place. Eventually, Stemers created a molecular scaff old that helped to correctly form the loop, and then created a second loop by forcing the end of the rope to close. This created the quasi[1] catenanes structure. The name is derived from the loosely intertwined, ring-like molecular structures called catenanes that were developed by French chemist Jean- Pierre Sauvage, Scottish chemist Sir Fraser Stoddard and Dutch chemist Ben Feringa. These three chemists won the 2016 Nobel Prize in chemistry for their work with catenanes.
In a continuation of their research, Stemers and team are hoping to add breakable bonds to the quasi[1]catenane structure that would allow the rings to be unlocked.



