Learn More About the Roche cobas Liat System
Point-of-care infectious disease testing now includes PCR-based procedures that can be performed in about 20 minutes and with minimal hands-on time.
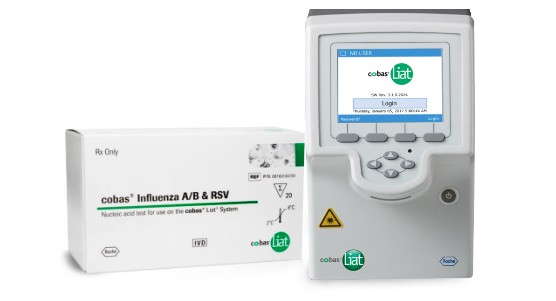
In just three steps, the Roche cobas™ Liat™ Assays and Analyzer automates the entire nucleic acid test (NAT) process: reagent preparation, target enrichment, inhibitor removal, nucleic acid extraction, amplification, real-time detection, and result interpretation.
- Helps physicians make more informed treatment decisions
- CLIA-waived for influenza A/B, RSV, and Strep A
- More tests under development
| Catalog Number | Description | ||
|---|---|---|---|
SARS-CoV-2 + Influenza A/B Multiplex Assay | Learn More | ||
Influenza A/B + RSV Multiplex Assay | Learn More | ||
SARS-CoV-2 Assay | Learn More | ||
Strep A Assay | Learn More | ||
C. Diff Assay Kit | Learn More |

By submitting your data, you agree to our Privacy Policy and will receive emails based on your preferences.
© Thermo Fisher Scientific, Inc. All Rights Reserved.