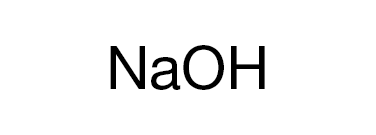
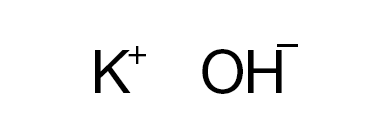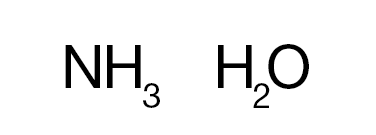Bases
Bases are usually thought of as the chemical opposite of acids. They are commonly defined as compounds that form aqueous solutions that taste bitter, have a high pH (>7), cause litmus paper to turn blue, are slippery to the touch, and react with acids to form water and salts.
Read More About BasesMilliporeSigma High-Purity Acids and Bases Brochure
Browse the high-purity MilliporeSigma lines of OmniPure and OmniPure Plus acids for trace metal analyses.
Honeywell Inorganic Trace Analysis Brochure
Learn more about high-purity Honeywell Fluka TraceSELECT acids, bases, solvents, and salts for trace metal analyses.
- Honeywell Inorganic Trace Analysis Brochure (468.9 KB)