Multi-Attribute Method in Therapeutic Protein Manufacturing Quality Control
The Multi-Attribute Method (MAM) is an emerging quality control process that couples liquid chromatography and high-resolution accurate mass (HRAM) mass spectrometry. MAM is used to monitor multiple product quality attributes simultaneously during the manufacturing of therapeutic proteins.
Therapeutic proteins are a unique class of large-molecule drugs with much more complex structures than small-molecule drugs. During their manufacturing and storage, these proteins may be subject to chemically or enzymatically generated primary structure modifications. Specifically, post-translational modifications (PTMs) can contribute to the overall heterogeneity that becomes part of the product quality attributes (PQAs) of therapeutic proteins. Modifications that can affect the drug’s efficacy and safety are classified as critical product attributes (CPQs) and must be monitored using various quality control (QC) processes.
One major advantage of MAM is that it can detect PTMs at the amino acid residue level, which is not possible using conventional profile-based methods of UV or fluorescence detection. The LC-HRAM-MS based MAM workflow involves initial enzymatic digestion of therapeutic protein to produce peptides of various length. This is followed by gradient separation using mobile phases with acetonitrile and water with either formic acid or trifluoroacetic acid as modifiers. The HRAM MS analysis of the eluted peptides is used to determine the sequence coverages that pinpoint the PTMs.
MAM must be robust and validated based on regulatory requirements to be useful for quality control methods in the biopharmaceutical industry. Moreover, the mobile phase solvents and reagents must be of high purity and without interfering contaminants. In a recently published study of MAM, more than 30 leading biopharmaceutical and LC-MS vendor labs cited chromatographic artifacts and contamination as two major causes of result abnormalities in peak detection.
Figure 1 shows an example of two control (blank) runs with mobile phases prepared from two different brands of LC-MS grade acetonitrile. Brand X has an elevated background that could compromise sensitivity and a repeating peak pattern that could severely interfere with peptide peak detection. The mass differences of 44 Da suggest PEG or PEG-like contaminants. All LC-MS grade solvents are not created equal, so solvents and reagents used for mobile phases must be evaluated before implementing MAM methods.
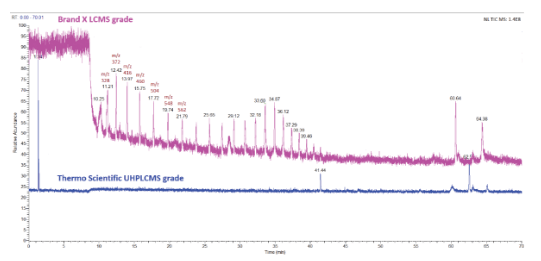
Figure 1. Control (blank) runs of an LC-MS MAM using two brands of LC-MS grade acetonitrile for mobile phases: brand X (top in pink) and Thermo Scientific (bottom in blue). The mobile phase is a binary gradient of 0.1% formic acid in water and 0.1% formic acid in acetonitrile. The m/z of seven peaks showing PEG-like +44 Da are labeled in brown.
Thermo Fisher Scientific offers two grades of chemicals that are fully tested for LC HRAM MS MAM and other LC-MS analyses. Fisher Chemical Optima grade LC-MS solvents and blends are precisely mixed for lot-to-lot consistency and tested using LC-MS methods. Thermo Scientific UHPLC-MS grade solvents meet even more stringent specifications for assays that require more sensitivity.
Optima LC-MS grade and UHPLC-MS grade solvents and blends have been fully tested for low organic impurities including PEG-like substances. In the newly introduced Thermo Fisher Scientific MAM 2.0 workflow, UHPLC-MS grade acetonitrile and water are specified for preparing the mobile phase for the initial system performance evaluation test (SET).

Content provided by:
