Improvements in Affinity Chromatography
Cytiva MabSelect PrismA is a next-generation protein A chromatography resin. It offers significantly enhanced alkaline stability and binding capacity for improved monoclonal antibody (mAb) processing.
The resin builds on the proven track record of MabSelect and MabSelect SuRe resins, but MabSelect PrismA has an optimized high-flow agarose base matrix and a genetically engineered protein A-derived ligand.
Key features include:
- Enhanced dynamic binding capacity (DBC) for high mass throughput of processed mAb per resin volume unit
- Alkaline stability enables efficient cleaning and sanitization using 0.5 to 1.0 M NaOH
- Dual sources for the agarose base matrix and protein A ligand for supply chain dependability
Since mAbs were first approved for commercial use in 1980s, they now represent the fastest growing segment of biopharmaceutical sales. Over the past 30 years, protein A chromatography resins and mAbs have seen annual productivity gains in the protein A step (more than 4.5 percent) and increased protein A binding capacity (more than 5.5 percent).1
Their high affinity for the antibody Fc region makes protein A resins an efficient mAb purification platform. The homology of the Fc region allows most mAbs to be purified using a standard approach, which significantly reduces process development time. This explains why nearly all commercially approved mAb manufacturing uses protein A capture as the first purification step.
Designed for High Productivity in mAb Capture
Most mAbs are sent for purification, which makes the protein A step very important. Increasing mAb titers means that cell culture feeds contain more impurities. The high nutrient load in the cell culture harvest, combined with the low alkaline resistance of protein A resin, creates an elevated risk of resin fouling and bioburden issues.
For efficient upstream batch purification, the resin capacity needs to match the mass of produced mAbs. Historically, the binding capacity of protein A resins has lagged behind ion exchange chromatography resins, requiring larger resin volumes and chromatography column sizes.
Due to the enhanced properties of both the protein A ligand and the base matrix design, MabSelect PrismA offers significantly increased binding capacity compared with its predecessor, MabSelect SuRe LX resin.2 For similar process setups and column sizes, the improved binding capacity of MabSelect PrismA enables significantly increased mass throughput per purification cycle compared with MabSelect SuRe LX.
With the increased binding capacity, the productivity of current chromatography columns and systems can be improved without costly capital expenditures, making more efficient use of existing manufacturing footprints. Alternatively, the increased binding capacity can be used to decrease the resin volume (and concomitantly the buffer consumption) required to achieve a given mass throughput.
MabSelect PrismA and Binding to Non-Fc Regions
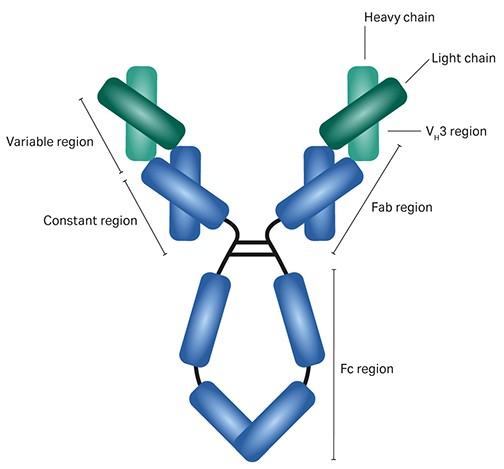
The binding of protein A mainly takes place between constant heavy chain domains — CH2 and CH3 — in the Fc region of the mAb. The protein A ligand in MabSelect PrismA has enhanced binding affinity for the VH3 sequence located on the variable heavy chain of the Fab region compared to its predecessors, MabSelect SuRe and MabSelect SuRe LX. MabSelect, MabSelect SuRe, and MabSelect PrismA interact to different degrees with VHH fragments.
These VHH fragments are unique to Camelids and are single-chain antibody mimics of the VH3 region of IgG with mutations at different positions. MabSelect PrismA has a similar binding pattern for the tested VHH sequences as the recombinant protein A ligand present in MabSelect and MabSelect Xtra resins, which provides new opportunities for purification of increasing molecular diversity of antibody fragments.
Improved Alkaline Stability Enables New Standards for Cleaning and Sanitization
Sodium hydroxide (NaOH) has gained popularity for bioprocessing, cleaning, and sanitization due to its efficacy, low cost, and ease of detection, removal, and disposal. Commonly, 1 M NaOH is used to clean and sanitize chromatography columns and resins. In addition to its ability to inactivate endotoxins and many microorganisms, NaOH can remove bound proteins, nucleic acids, and lipids from the resins. Fouled resins can increase cross-contamination risk, which my negatively impact DBC over time.3 Historically, protein A chromatography resins have been sensitive to NaOH due to the limited alkaline stability of the protein ligand.
Later generations of protein A resins exhibit improved alkaline stability and have recommended cleaning procedures using 0.1 M NaOH, with the option of occasionally using 0.5 M NaOH. Given that protein A capture is the first purification step and therefore exposed to the crudest feed and highest impurity loads, the use of weak CIP agents has been highlighted as a challenge and risk with using this technique.
With enhanced alkaline stability of its protein A ligand, MabSelect PrismA meets this challenge.4 The ligand was developed using high-throughput screening to identify amino acids sensitive to alkaline degradation and substitution of these amino acids with more stable ones. The final construct constitutes a hexamer of the engineered domain. Highly pure ligand is immobilized to the agarose base matrix via a chemically stable thioether linkage. The enhanced alkaline stability enables efficient cleaning of the resin using 0.5 to 1.0 M NaOH over many purification cycles.5
References
- Bolton G. R. and Mehta, K. K. The role of more than 40 years of improvement in Protein A chromatography in the growth of the therapeutic antibody industry. Biotechnol. Prog. 32, 1193–1202 (2016). https://doi.org/10.1002/btpr.2324
- Application note: Capacity and performance of MabSelect PrismA protein A chromatography resin. GE Healthcare, KA1965231408AN (2018).
- Zhang J. et al. Maximizing the functional lifetime of Protein A resins. Biotechnol. Prog. 33, 708–715 (2017). https://doi.org/10.1002/btpr.2448
- Whitepaper: Efficient cleaning-in-place methods for protein based antibody affinity chromatography resins. Cytiva. KA1619220218WP (2018).
- Application note: Lifetime performance study of MabSelect PrismA during repeated cleaning-in-place cycles. GE Healthcare, KA1061080618AN (2018).