Five of the Most Useful Transformations in Modern Organic Synthesis
Since World War II, the monumental efforts of the pharmaceutical industry in pursuit of new biologically active molecules have been a major force in the evolution of synthetic organic chemistry. Incredible creativity has produced decades of chemical knowledge, with new discoveries from a toolbox of efficient reactions. This has enabled the synthesis of complex, multi-functional molecules.
During the past 25 years, several scientists have received the Nobel Prize for synthetic methodologies that have changed the way chemists approach molecular design. Palladium-catalyzed cross-couplings, asymmetric hydrogenation, epoxidation, and olefin metathesis have led to the development of completely new synthetic strategies. These have played a key role in the discovery and synthesis of L-Dopa,1 Ledipasvir, Losartan,2 Atorvastatin, and other new and important drugs.
Despite this, modern synthetic organic chemistry is often perceived as a more-established discipline with limited innovation. While its role remains the foundation of new drug discovery and development, R&D efforts have moved to other areas,3, 4, 5 mostly to the interface of biology and chemistry. From this perspective, chemical strategies have clearly evolved from the end of the 1900s to present day.
Brown and Boström6 analyzed medicinal chemistry literature in 1984 and 2014 and found that a limited number of reactions dominated the chemical landscape. With few exceptions, the most common reactions of 1984 were still being used in 2014. Carbon-carbon cross-coupling was the only new entrant, and an increase in amide bond formations and decrease in heterocyclic synthesis were the only other significant changes.
The five most common reactions, representing over half of the total number of references, are:
- Aromatic nucleophilic substitution (SNAr)
- Alkylation of amines/nucleophilic substitution of alkyl halide
- Amine protection and deprotection
- Amide synthesis
- C-C cross-coupling
Aromatic nucleophilic substitution and the nucleophilic substitution of alkyl halides are “historical” chemical transformations. They are well-known, robust, and efficient. The first has been used since the early 1950s and is the method of choice for functionalizing aryl compounds. However, it is being replaced in favor of the more robust Pd-catalyzed Buchwald-Hartwig C-N coupling for synthesizing aromatic amines.
The second reaction, alkylation of amines, remains a primary tool for synthetic chemists despite its release of a halide side product, which is not environmentally friendly. It also requires relatively high temperatures.
Because amine functionality in bioactive molecules is very common, amine protection and deprotection reactions are widely utilized in synthetic chemistry. The method of choice is the mild and high-conversion carbamate formation, a reaction between the amine and the BOC (tert-butyloxycarbonyl) protecting group, chemically a di-tert-butyl dicarbonate (Boc2O). The deprotection of the protected amine is a simple carbamate hydrolysis that takes place in acidic conditions.
These first three reactions have remained unchanged. Their simplicity, broad substrate scope, and robustness make them extremely practical and offer flexibility for synthesizing multi-functional compounds.
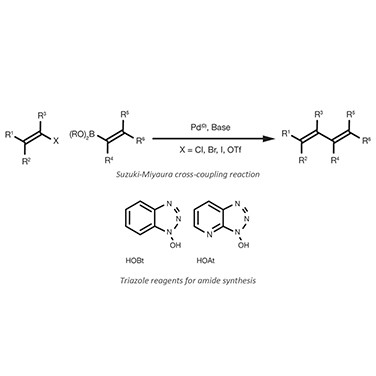
The fourth reaction, amide synthesis, includes a long-time standard: the Schotten-Baumann reaction or acylation of amine by acyl chlorides. It proceeds rapidly and results in high conversion at room temperature, but suffers from the instability of the acyl chlorides, hazards related to their decomposition, and the release of a hydrochloric acid by-product.
Progress in the field of peptide synthesis presented an opportunity to address these shortcomings and inspired a significant update to this chemistry. The use of carbodiimides and, more recently, hydroxybenzotriazole, aminium/uronium, or phosphonium salts to generate highly activated ester intermediates has completely displaced the use of acyl chlorides and the Schotten-Baumann reaction.
The fifth and final reaction addresses a major organic synthesis challenge, the formation of C-C bonds. Carbon-carbon cross-coupling, the newer entrant in the ranks, represents one of the biggest revolutions in organic chemistry and has rapidly become ubiquitous in fine chemical synthesis. The discovery by Akira Suzuki, Ei-Ichi Neghishi, and Richard Heck won the Nobel Prize for Chemistry in 2010.
Among the various types of cross-coupling, the Suzuki-Miyaura7 reaction — usually called “Suzuki coupling” — arguably has the broadest utility and applicability. The Suzuki chemistry is based on the Pd(0) catalysed coupling of an aryl or vinyl halide with an aryl or vinyl boronic acid. This reaction was discovered at the end of the 1970s and has gained greater popularity since 2000, almost completely displacing the use of organozinc and organostannane compounds.
These top five reactions tell us a story of tradition and exemplify a constant and often subtle flow of innovation. Three of them have fundamentally remained the same, one evolved significantly in its application protocol, and one represents a completely new transformation.
New discoveries like C-C cross-coupling may provide leaps forward and profoundly change the chemical landscape. But innovation is more often driven by organic changes in applications and reaction conditions8 inspired and driven by research in related fields. The aromatic nucleophilic substitution and BOC protection/deprotection haven’t changed, while amide synthesis has benefited from progress in peptide chemistry, where it offered a solution to an existing problem.
Robustness, flexibility, broad substrates, and reaction condition scope unify these reactions and explain their popularity.9 Nonetheless, the importance of other, more niche transformations cannot be understated. While not as frequently used as the “top five,” they still play key roles in a chemical space that would otherwise be inaccessible.10
The commercial availability of starting materials, building blocks, and reagents will continue to play a significant role in the development of the field. Thermo Fisher Scientific’s Acros Organics and Alfa Aesar brands offer a comprehensive portfolio of fine chemicals, reagents, and chemical essentials. Find products in everyday sizes and bulk quantities to support synthetic organic chemists from the early stages of R&D to advanced process chemistry and production.
Visit fishersci.com/buildingblocks or fishersci.ca/buildingblocks to find the right products for your work.
1. W. S. Knowles, (2002). Angew. Chem. Int. Ed. 41 (12): 1998–2007
2. R. D. Larsen et al. J. Org. Chem. 59, 6391 (1994)
3. P. Ball, Chemistry: Why synthesize? Nature 528, 327–329 (2015)
4. G. M. Whitesides, Angew. Chem. Int. Ed. 54, 3196–3209 (2015)
5. T. Laird, Org. Process Res. Dev. 14, 749 (2010)
6. J. Med. Chem. 2016, 59, 4443−4458
7. Chem. Commun. 1979, 20 (36): 3437-3440. Chemical Reviews 1979, 95 (7): 2457–2483. J. Chem. Soc., Chem. Commun. 1979, 0 (1): 866-867.
8. J.G. Donaire et al., J. Am. Chem. Soc., 140, 355-361 (2018)
9. J.Boström, et al., Nat Rev Drug Discov. 17, 709–727 (2018)
10. K.R. Campos et al., Science 363, 244 (2019)