Microplate-Based Cell Viability Assays Using Absorbance, Fluorescence, or Luminescence Detection
As many as one third of all putative drugs fail from toxicity issues, which contributes to the high cost of pharmaceutical R&D, particularly if toxicity is proven in clinical trials. Thus, toxicity testing of small molecules is typically performed pre-clinically using cell-based in vitro assays as a first step to identifying any toxicity issues.
There are numerous assay technologies available commercially for probing cellular viability where adverse effects cause a drop in assay signal. These assays are typically designed for workflows that use microplates and optical detection typical of microplate readers. In this technical bulletin, we demonstrate three of these cell viability technologies that use absorbance, fluorescence, or luminescence detection all measured on the low-cost BioTek Synergy LX Multi-Mode Reader.
Materials and Methods
Toxicity Experiments
Human prostate cancer cell line (PC-3) cells were cultured in Hams F12K Nutrient mixture supplemented with 10% fetal bovine serum and penicillin-streptomycin at 37°C in 5% CO2. Cultures were routinely trypsinized (0.05% Trypsin-EDTA) at 80% confluence.
Cells were plated into Corning 3904 black-sided, clear-bottom 96-well microplates using DMEM/F12 media without phenol red to create 30,000 total cells per well in 100µL. After 24 hours to allow for attachment, the cytotoxic compounds cycloheximide and tamoxifen were added to the cultures at various concentrations in 100µL. After 24 hours of exposure, 100µL of cell media was removed and diluted reagent added according to the outlines below.
Colorimetric MTT Assay
10µL of 12mM Vybrant MTT Cell Proliferation Assay Reagent (Cat. No. V13154) was added. Reaction was allowed to proceed for four hours at 37°C in a humidified environment. The reaction was stopped and the insoluble formazan product resolubilized by the addition of 100µL of 10% SDS in 0.01 N HCl solution and incubation for 16 hours at 37°C in a humidified environment. Absorbance at 570nm was measured with a Synergy LX Multi-Mode Reader using the dedicated UV-Vis monochromator.
Fluorometric Calcein AM Assay
100µL of 5µM Calcein AM (Cat. No. C34852) was added. Cells were incubated at 37°C for 30 minutes, after which fluorescence was determined with a Synergy LX Reader using a green fluorescence cube. The green fluorescence cube was configured with a 485/20 excitation filter, a 528/20 emission filter, and a 510nm cut off dichroic mirror.
Luminescent CellTiter-Glo Assay
100µL of reconstituted CellTiter-Glo Cell Viability reagent (Cat. No. PR-G7572) was added. The plate was shaken on an orbital shaker for two minutes, then incubated for 10 minutes in the dark at room temperature within the Synergy LX Reader prior to luminescence determination using a dedicated luminescence cube. This cube allows direct passage of light from the microplate well to the PMT detector. The default settings for PMT gain (135) and collection time (one second) were used.
Cell Titration Experiments
For cell titration experiments, a range of PC-3 cell concentrations were used up to 36,000 cells/well. After 24 hours, reagent was added according to the manufacturer’s specifications and read on the Synergy LX Reader using the detection modes described above.
Results and Discussion
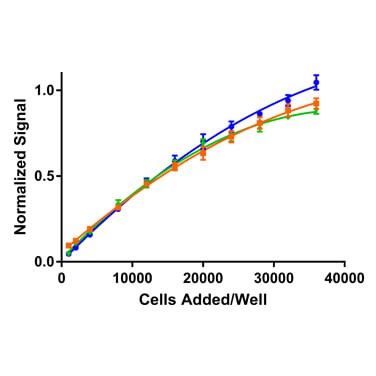
Each of the reagents tested probes cell health, albeit in different ways. The colorimetric MTT assay relies on endogenous NAD(P)H oxidoreductase enzymes to reduce MTT to a colored formazan dye. The Calcein AM reagent is converted by endogenous cytoplasmic esterases from a non-fluorescent to a fluorescent compound. CellTiter-Glo is based on the quantification of ATP present, which indicates the presence of metabolically active cells. Thus, for each reagent, one would expect greater assay signals as more viable cells are present. This is evident in Figure 1, which demonstrates cell titrations for each of the detection technologies tested.

In the case of a toxicity experiment, in which cell health is probed after the addition of small molecule compounds, one would expect a reduction in signals for each of these detection technologies should the compound display toxicity. The extent of reduction depends on how the specific enzymes involved in generating signal are affected or the metabolic activity of the cells in the case of ATP measurement. Figure 2 illustrates these impacts when the cells are treated with cycloheximide and tamoxifen.

Cycloheximide is used widely in biomedical research to inhibit protein synthesis in eukaryotic cells studied in vitro. Its toxic effect is plainly seen in Figure 2, where for each technology a significant drop in signal is evident with increasing dose of the compound. For each technology, the dose at half the maximum signal drop is approximately 0.1µM. Note also that the extent of cytotoxicity appears to be greater for the detection technologies based on cytosolic enzyme turnover (MTT and Calcein AM) compared to ATP measurement (CellTiter-Glo). Conversely, tamoxifen demonstrates little toxicity over its full dose range, which is not unexpected for this medication widely used to treat breast cancer.
Conclusions
The Synergy LX Multi-Mode Reader is a low-cost solution for the most common detection technologies used in life sciences research, including absorbance, fluorescence, and luminescence detection. Here we demonstrated its utility to quantify cellular toxicity using widely used cell viability assays that employ these common detection modes.
Content provided by: