Organometallics: Vital and Versatile Compounds
The Importance and Versatility of Organometallic Chemistry
Organometallics are versatile and widely used compounds that play a vital role in chemical research and industrial applications.
Organometallic compounds contain at least one bond between a carbon atom and the atom of a metal element. The electropositive character of their metals makes organometallic compounds highly reactive and vital to many chemical syntheses. This broad category includes chemicals with significantly differentphysical and chemical properties, which are used as homogenous catalysts, istoichiometric reagents, and in many other applications.
Early Organometallic Reagents
One of the first organometallics to be identified was diethylzinc, discovered in 1848 by the British chemist Edward Frankland. By combining zinc metal with ethyl iodide, Frankland produced a colorless liquid that reacted violently with water and ignited upon contact with air.
Fifty years after Frankland’s discovery, organometallic chemistry and its practitioners were still creating and identifying compounds. French chemist Victor Grignard developed the novel organomagnesium reagents in the 1900s, which are still among the most important in modern chemistry. Now named Grignard reagents, their formula is RMgX, where X is a halogen and R is an alkyl or aryl group. Grignard was awarded the 1912 Nobel Prize in Chemistry for his pioneering work.
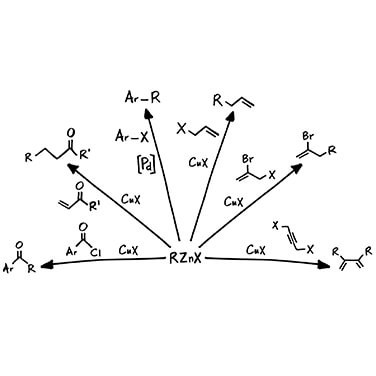
Applications of Grignard and Organozinc Reagents
A significant use of organometallic compounds is in the formation of new carbon–carbon bonds. Both Grignard reagents and organozincs (specifically organozinc halides) are an excellent source of nucleophilic carbon atoms because of the polarity differences within the metal–carbon bond. These nucleophilic carbon atoms react with electrophilic carbon to produce a new carbon–carbon bond, which is a very convenient method of assembling compounds from smaller precursor molecules.
In these reactions, the organozincs are less reactive than Grignard reagents. This is an advantage for certain sensitive reactions that require higher functional group tolerance, but this low reactivity means that organozincs often need the help of additives or catalysts.
Because Grignard and organozinc reagents are used in highly sensitive reactions, the quality of organometallic reagents is crucial. Inferior reagents may cause incomplete or failed reactions, which reduces product yields. Selecting appropriate reagents for complex stereochemical syntheses also minimizes the potential for racemic mixtures.
Grignard and organozinc reagents from the Acros Organics and Alfa Aesar brands provide consistent stereochemical and high-yield results to help you reach your goals.
Other Organometallic Reagents
In addition to Grignard reagents and organozinc compounds, organolithium, organoaluminum, and organotin reagents have proven useful for a number of applications.
During the 1950s, German chemist Karl Zieglar reported that a combination of titanium tetrachloride [TiCl4] and organoaluminum chloride [Al(C2H5)2Cl] formed an excellent catalyst for producing polyethylene. Later, Italian scientist Giulio Natta used titanium (III) chloride [α-TiCl3] in combination with triethylaluminum [Al(C2H5)3] to produce the first crystalline isotactic polypropylene. These organometallics became known as Zieglar-Natta catalysts and are still used in the commercial manufacture of various polyolefins. Zieglar and Natta were awarded the 1963 Nobel Prize in Chemistry for their organic chemistry contributions.
Zieglar, along with Georg Wittig and Henry Gilman, also pioneered the use of organolithium reagents as alternatives to Grignards. Organolithium reagents share many of the same characteristics, and can be used in the same reactions as Grignard reagents but react more quickly and produce greater yields.
Organometallic innovations continued throughout the 20th century, including these key developments:
- The Heck reaction showed that organopalladium catalysts can form intricate substituted alkenes
- Cyclobutadieneiron tricarbonyl compounds were discovered
- Roald Hoffmann and Kenichi Fukui won the 1981 Nobel Prize in Chemistry for the creation of the Woodward- Hoffman Rules, which predict the stereochemical outcomes of pericyclic reactions.
Unparalleled Reagent Options
Organometallics are consistently in demand for many applications, and the Acros Organics and Alfa Aesar portfolios offer a broad range of organometallic reagents for use in your laboratory.
Whether you’re looking for enhanced organometallic reactivity, ease of workup, or reduced environmental impact solutions, our portfolio and expertise can support your chemical syntheses and research applications.
High-quality Acros Organics and Alfa Aesar synthetic reagents are ready to ship in multiple grades, specifications, and pack sizes, and many are available in AcroSeal packaging.